Abstract
Background: People with mild or moderate hemophilia A (HA) constitute 40-52% of all people with HA; nonetheless, they are a poorly characterized population in real-world data and published literature (Witkop et al. Blood 2021). To better understand this population, we used a novel platform, PicnicHealth, which integrates real-world data from patients' medical records and patient-reported outcomes, including bleeding occurrence, treatment for bleeds, and pain (Skinner et al. Blood 2021; Witkop et al. Blood 2021). We aim to study the epidemiology of mild and moderate HA and evaluate the effects of various demographic variables and the COVID-19 pandemic on use of healthcare facilities.
Methods: People with HA (PwHA) have been enrolled into the PicnicHealth online record management platform since June 2020. Inclusion criteria for this cohort with mild or moderate HA were based on baseline factor (F)VIII activity level (mild, >5-50%; moderate, 1-5%). Records were collected from all known healthcare providers and facilities, including hematology, primary care, and other specialty care, in addition to laboratory reports, imaging reports, pathology, and any inpatient or outpatient encounter since the diagnosis. Descriptive statistical analyses were performed to summarize cohort characteristics.
Results: As of June 2022, there were 113 PwHA enrolled in PicnicHealth who met the inclusion criteria for this analysis, of whom 68 (60.2%) had mild HA and 45 (39.8%) had moderate HA. In total, 90 (79.6%) participants were male and 23 (20.4%) were female. The majority identified as 'White' (n=77; 68.1%), followed by 'More than one race or unreported' (n=25; 22.1%), 'Black or African American' (n=8; 7.1%), and 'other race' (n=3; 2.7%). The median age at diagnosis was 12.5 years (Q1, Q3: 1.96, 29.8). Only 8 participants (7.1%) had evidence of FVIII inhibitors during their lifetime. The median (Q1, Q3) Hemophilia Joint Health Score was 4.00 (3.00, 9.00) and the median (Q1, Q3) annualized bleed rate was 0.17 (0, 3.63). In terms of treatment, in the 2 years prior to enrollment, 36 (31.9%) participants had received FVIII prophylaxis, 73 (64.6%) had received FVIII on demand, and 10 (8.8%) had received emicizumab (Table 1).
In the 2 years prior to enrollment, participants with mild or moderate HA visited a healthcare facility a median (Q1, Q3) of 7 (4, 11) times and a hematology practitioner a median (Q1, Q3) of 2 (1, 3) times. The majority of the visits to hematology practitioners were to medical doctors (93%), with a smaller number to nurse practitioners or allied health professionals (7%). There was no notable difference in the median number of visits to healthcare facilities or hematologists according to disease severity, race, or inhibitor status. The five states with the highest number of healthcare facility visits by hemophilia patients were California (24.7%), Indiana (7.1%), Michigan (5.9%), Texas (5.9%), and Virginia (4.3%).
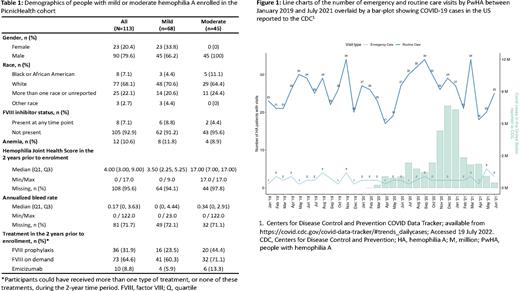
Finally, we assessed the impact of the COVID-19 pandemic on participants' visits to healthcare facilities. Between January 2019 and July 2021, there were no apparent changes to emergency care visits. For routine care visits, there have been variations since the pandemic began, which may be attributable to lockdown restrictions, although the number of these does not strictly correlate with COVID-19 case numbers (Figure 1).
The study is limited by potential bias resulting from participant reporting and the retrospective nature of data collection.
Conclusions: Our study provides valuable insights into the characteristics and utilization of healthcare of the under-represented cohort of people with mild or moderate HA. We found notable variation in the geographical distribution of healthcare facilities and visits by PwHA across different states. As expected, routine visits by patients fluctuated during the COVID-19 pandemic. The data generated from this study may aid planning for, and delivery of, the most appropriate and effective care for this group of patients.
Disclosures
Sharathkumar:University of Iowa Clinical Committee: Membership on an entity's Board of Directors or advisory committees; Genentech, Takeda, CSL: Honoraria; Amgen, CDC/ATHN/HRSA: Research Funding; University of Iowa: Current Employment. Jiang:Roche Products Ltd: Current Employment; Whittington Health NHS Trust: Ended employment in the past 24 months. Hiew:Roche Products Ltd: Current Employment; University Hospital Dorset: Ended employment in the past 24 months. Siadimas:F. Hoffmann-La Roche AG: Current Employment, Current equity holder in private company, Research Funding. Nissen:F. Hoffmann-La Roche Ltd: Current Employment, Current holder of stock options in a privately-held company. Hanson:PicnicHealth: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Cibelli:PicnicHealth: Current Employment, Current holder of stock options in a privately-held company. Moreno:F. Hoffmann La Roche Ltd.: Current Employment, Current equity holder in publicly-traded company, Honoraria. Wharton:Roche Products Ltd: Current Employment, Current holder of stock options in a privately-held company. Weyand:Bayer: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech, Inc: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Spark: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.